
Press Release
Press Release
October 28, 2022
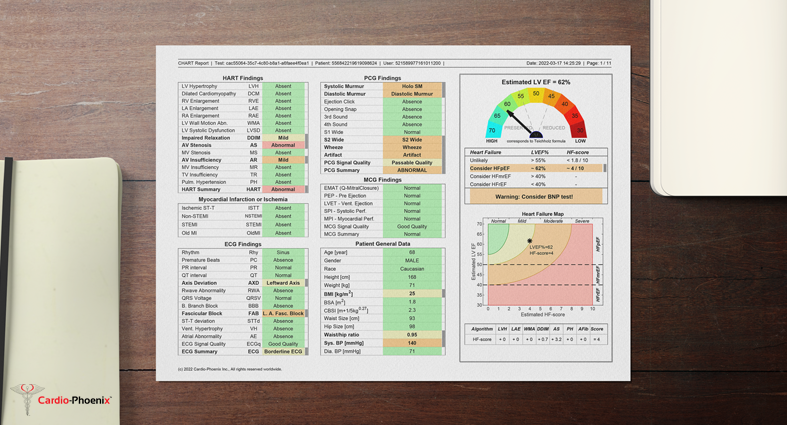
Cardio-HART Report Sample
Example Sample of Cardio-HART report for a patient with HFpEF.
Download PDF
https://www.cardiophoenix.com/files/Documents/CHART_report_sample.pdf

Press Release
March 7, 2022
Novel Technology Improves GP referral decisions to cardiology
Cardio-HART is the clinician’s AI-powered heart diagnostic assistant. Early detection of heart disease can prolong or increase a patient's quality of life. In addition, it will reduce the burden on the health system and create better communication between GPs and cardiologists.
Open Heart magazine published a study “Novel tech throws knock-out punch to ECG improving GP...
Press Release
December 16, 2020
Cardiac telemedicine, CHART device arrives that detects 95% of all heart diseases
CE-marked approval could change the work of specialists and allow them, at the end of the pandemic, to manage the wave of new heart patients
After fifteen years of work and CE Mark approval, the Cardio-HART™ or CHART revolutionary cardiac diagnostic device has arrived on the market. Just in time to address all Covid-19 associated heart...

Press Release
December 16, 2020
Cardiac Device for Telehealth Receives CE Certification
Unlike other remote monitoring devices, CHART is approved for use in Primary Care, from the first contact the patient has with the health system.
Cardio-HART™ or "CHART" is an innovative cardiac diagnostic device that has been CE-approved, just in time to address the legacy of COVID-19-associated cardiac disease and help reduce the overwhelming waiting lists. What...
Press Release
August 20, 2020
Cardio-HART™ - a Breakthrough Cardiac-Telemedicine™ device, now CE-Marked
Just in time to meet the consequences of COVID-19-caused heart disease, CHART has obtained CE Mark certification for use in the EU.
Telemedicine is the future of patient visits to the doctor, especially in this pandemic as it helps maintain social distancing safety guidelines. This will be critical in the face of the feared second wave...
Press Release
January 9, 2019
AI innovations in primary care
for immediate release
On December 14th, Cardio-Phoenix Inc. received a 510 (k) clearance for its Cardio-TriTest™ v6.5 medical device from the U.S Food and Drug Administration (FDA). This is the second 510k the company has received, the first being issued June 3rd, 2015.
The Cardio-TriTest™ (CTT) device is a non-invasive medical device that collects 3 different types...

Press Release
May 28, 2018
Cardio Phoenix is now ISO13485:2016 certified
Cardio-Phoenix Inc. - Now ISO 13485:2016 Certified
Cardio–Phoenix Inc. is proud to announce that it has received the coveted ISO 13845:2016 certification for the Design and Development of non-invasive diagnostic medical devices.
ISO certification of Cardio-Phoenix demonstrates the company’s quality commitment to developing advanced products and novel technology for the early detection of a broad range of...
